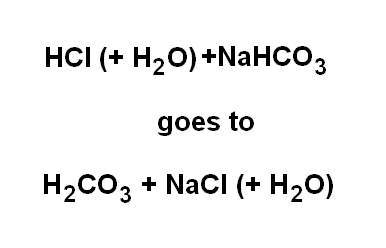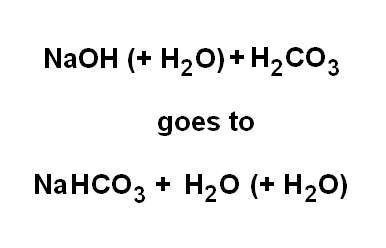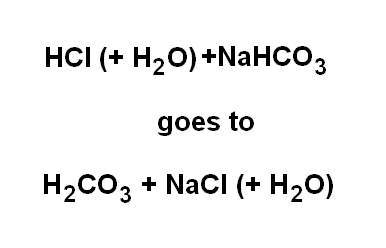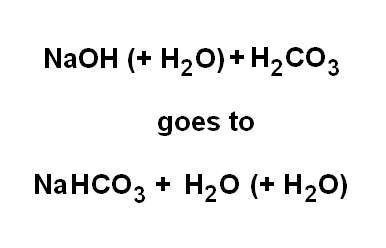The following is a possible explanation for the cancer/water correlation:
Arterial blood has a pH in the range 7.35 to 7.45 which is slightly more alkaline than 7.0 or neutral. If the pH falls below 7.35 it is called acidosis, and if the pH is raised above 7.45 it is called alkalosis.
Arterial blood outside this very narrow range has a dramatic effect on metabolism.
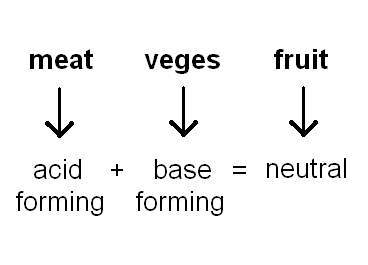
The foods we eat can be either acid or base forming. For example, acid forming foods are meat and eggs which contain chlorine, phosphorous and sulphur, while base (alkali) forming foods are vegetables which contain calcium, magnesium, potassium and sodium.
Acid buffer cancer hypothesis
Background to acid/base buffering:
Buffering:
As the food we eat can be either acid or base forming, the pH of arterial blood could theoretically be forced outside the 7.35 - 7.45 range. However, buffering is a process used by the body which employs ion exchange to moderate the pH and keep it within the required range.
There are two main types of buffer systems: chemical and physiological. Chemical buffers are the first defense against hydrogen ion changes and act immediately. Physiological buffers are the secondary defence and show an effect within 1-2 minutes or up to 24 hours.
Chemical buffers:
Chemical buffers are buffer pairs comprising a weak acid and a salt of that acid. Examples are bicarbonate pairs, phosphate pairs, hemoglobin pairs and the plasma-protein pair.
Using the bicarbonate buffer as an example, if a strong acid such as hydrochloric acid is added to solution then the hydrogen will dissociate to form hydrogen ions as hydronium and the buffer, sodium bicarbonate, exchanges its sodium with the
The ion exchange substituting a strong acid with a weak acid is the buffering action which moderates the pH.
Similarly, the addition of a strong base to solution will result in an exchange of the buffer hydrogen ion with the base positive ion to form sodium bicarbonate and water:
Physiological buffers:
If chemical buffers cannot sufficiently and immediately stabilise pH then the respiratory system will act in 1-2 minutes. Increased respiration removes more carbon dioxide from the venous blood entering the lungs and lowers the amount of carbonic acid (and therefore there are fewer hydrogen ions) in the arterial blood leaving the lungs. Decreased respiration has the opposite effect in response to raised pH.
H5O2+, which cannot be buffered by the buffer systems above. Rather than having a single hydrogen proton to deal with, there would be a proton triplet instead.
© Stephen G. Butcher (Posted 30/04/08)
(Revised 30/04/08
So a balanced diet of meat and veges makes sense in maintaining an acid/base balance!
In this way there is a recycling process of sodium bicarbonate to carbonic acid, carbonic acid to bicarbonate, and so on.
A similar process or recycling occurs with vitamin C, ascorbic acid, which can release hydrogen ions to bond with hydroxyl ions to form water, and after releasing two hydrogen ions forms dehydroascorbate which can accept hydrogen ions, and so on.
Finally, the kidneys exchange sodium and hydrogen ions to normalise pH within a 24 hour period. For instance, if blood pH decreases because of excess hydrogen ions (making it acid), then the kidneys can reabsorb a sodium ion from the urine in exchange for a hydrogen ion which it needs to get rid of,
Homeostasis:
When the three parts of the buffering system work together, homeostasis of the blood is maintained, i.e. blood pH is kept within the range required for the body to function correctly. Thus it should also be understood that a balanced diet of meat and vegetables, and fruit, with exercise and sufficient fluid intake are sensible ways to help the body maintain homeostasis.
Hydronium ions hypothesis:
If this is the case, a possible scenario of events would be that a gylcine in DNA takes on hydrogen triplets to form valine to act as a buffer. The now defective cell relies on destruction.
Cell destruction is only possible up to a certain number of replications, beyond which the change in DNA might be viewed as an adaption. Because the glycine has changed to a valine, the increase in hydrogen protons has increased cell metabolism such that the threshold of the minimum number of replications required for the cell to be considered adapted rather than defective is reached much sooner and before the immune system can intervene.
H+ ion to form carbonic acid, which is a weak acid, and salt:
The background so far has dealt with hydrogen ions, and buffering, when the ion
is in the hydronium form H3O+ where a single hydrogen proton is attached to a water molecule.
My hypothesis is that it may be possible that the series inductance and capacitance in conductive water pipe may result in another form of hydronium,